App : https://arkajitmandal.github.io/ExtendedHuckel/
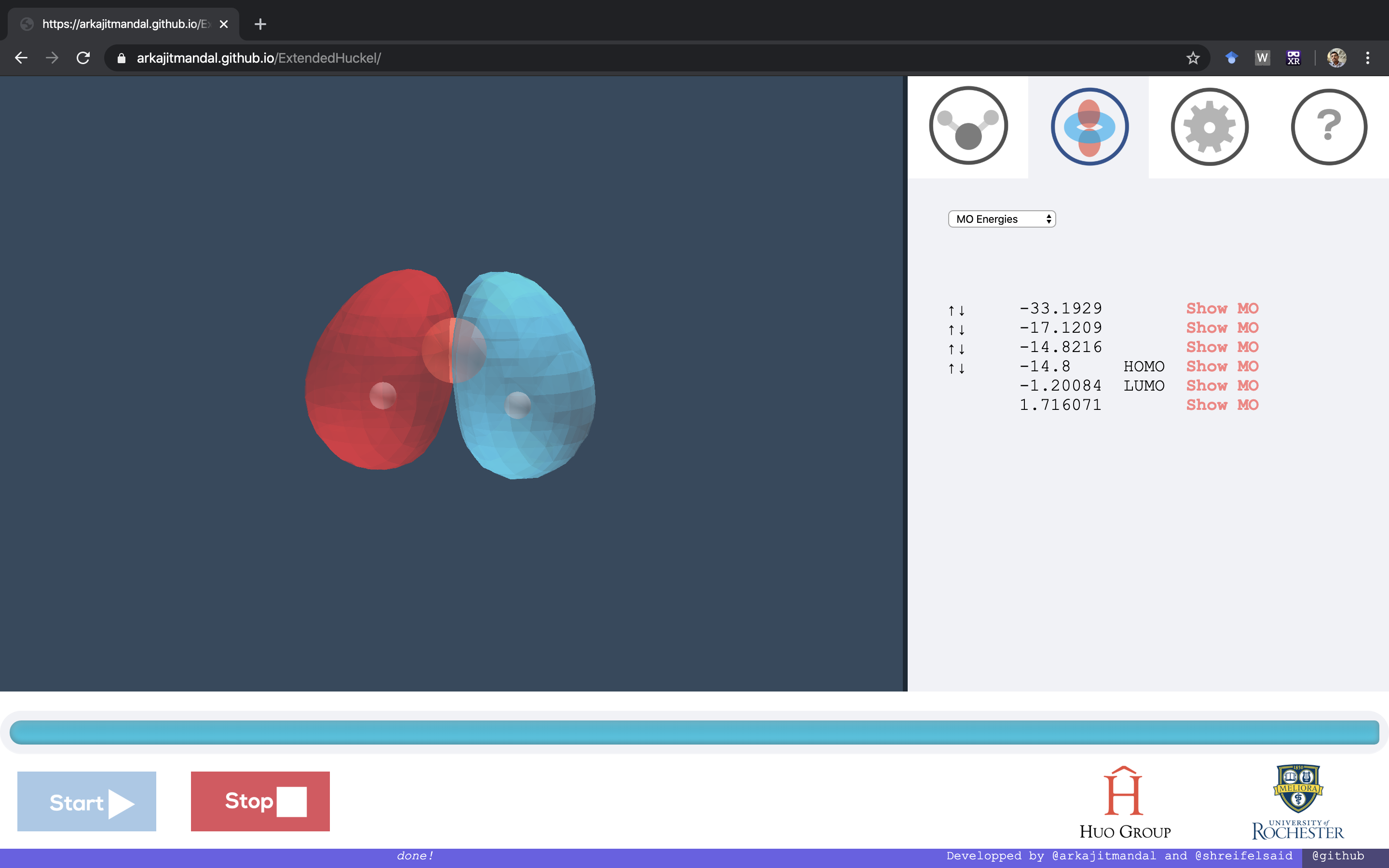
This is an app that allows students to obtain molecular electronic structure, i.e. how the atomic electrons make up molecular orbitals and how the corresponding wavefunctions look like. This will allow students to understand the basic chemical theories from a quantum mechanical standpoint. This uses an approximate semi-empirical theory called the Extended Huckel Theory.
To get started, one can write the molecular geometry (in Angstroms) in the text input box on the right side of the app. These geometries are available online in a number of different databases like http://www.chemspider.com/Chemical-Structure.937.html?rid=80ddc084-3622-43c5-8e0a-39376ff102f5. For example copy paste the following geometry of a molecule called Benzene (C6H6)
H -0.21463 0.97837 0.33136
C -0.38325 0.66317 -0.70334
C -1.57552 0.03829 -1.05450
H -2.34514 -0.13834 -0.29630
C -1.78983 -0.36233 -2.36935
H -2.72799 -0.85413 -2.64566
C -0.81200 -0.13809 -3.33310
H -0.98066 -0.45335 -4.36774
C 0.38026 0.48673 -2.98192
H 1.14976 0.66307 -3.74025
C 0.59460 0.88737 -1.66708
H 1.53276 1.37906 -1.39070
After copying the above structure click Start and then the outputs will be show on the right side of the app. Click Show MO (molecular orbital) to show the molecular orbital with a molecular electronic wavefunctions.
Try this another molecule called water (H2O)
O 0.00000 0.00000 0.0
H 0.00000 0.75545 -0.47116
H 0.00000 -0.75545 -0.47116
Or alternately you can use a .xyz file (https://en.wikipedia.org/wiki/XYZ_file_format). You can find some here: http://materia.fisica.unimi.it/manini/dida/structures.html
Here’s a video of me using the app.
https://twitter.com/arkajitmandal/status/1210642826695974912